Highly efficient direct large-scale stoichiometric aldol reactions catalyzed by a new diamine salt in the presence of water†
Abstract
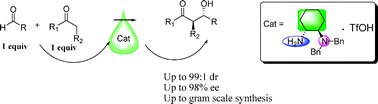
A new type of primary–tertiary diamine salt prepared can be used as a catalyst for direct aldol reaction and can display high yields, diastereoselectivities (up to 99 : 1), and enantiomeric excesses (up to 98%), by using stoichiometric amounts of cyclic ketones and various aryl aldehydes in the presence of water. This new catalyst can also be efficiently applied in large-scale reactions with the enantioselectivity being maintained at the same level, which indicates the potential for applications in industry.

 Please wait while we load your content...
Please wait while we load your content...