N-Tosylhydrazones: versatile reagents for metal-catalyzed and metal-free cross-coupling reactions†
Abstract
Transition metal-catalyzed cross-coupling reactions have been established as one of the most powerful tools for the construction of C–C and C–X bonds. In this context, the development of novel metal-catalyzed cross-coupling processes that do not require stoichiometric organometallic reagents is particularly attractive. Recently, N-tosylhydrazones have emerged as a new type of versatile coupling partners for transition metal-catalyzed cross-coupling reactions as well as metal-free cross-coupling reactions, and have attracted increasing attention. This tutorial review summarizes recent important developments in this area with N-tosylhydrazones as versatile coupling partners.
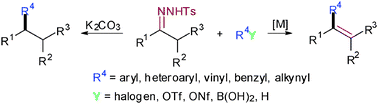

 Please wait while we load your content...
Please wait while we load your content...