Kinetic study of the reaction of vanadium and vanadium–titanium oxide cluster anions with SO2
Abstract
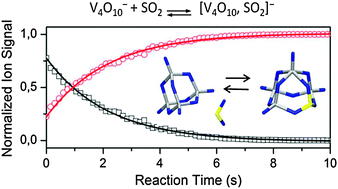
The reactivity of mass-selected V4O10− cluster anions towards sulphur dioxide is investigated in an ion trap under multi-collision conditions. Gas phase reaction kinetics are studied as a function of temperature (TR = 150–275 K). The binding energy of SO2 to V4O10− is obtained by analyzing the experimental low pressure rate constants, employing the Lindemann energy transfer model for association reactions in conjunction with statistical RRKM theory. In addition, infrared multiple photon dissociation spectroscopy is used in conjunction with density functional theory for the structural assignment of the [V4O10−, SO2] complex, revealing a square pyramidal structure with the SO2 molecule incorporated in the vanadium oxide framework. Energy profiles are calculated for the reaction between V4O10− and V6O15− with SO2. Whereas the transition structures along the reaction pathway of V4O10− with SO2 have energies below those of the separated partners, the reaction of V6O15− with SO2 proceeds via a transition structure with energy higher than the educts. The role of cluster size and composition is investigated by studying the reaction kinetics of larger (V6O15− and V8O20−) and titanium doped (V3TiO10− and V2Ti2O10−) vanadium oxide clusters with SO2. The observed cluster size and composition dependencies are discussed.

 Please wait while we load your content...
Please wait while we load your content...