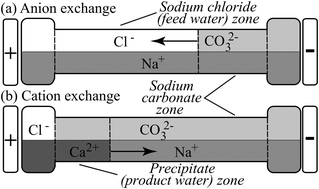
We demonstrate and analyze a novel desalination method which works by electrophoretically replacing sodium and chloride in feed salt water with a pair of ions, calcium and carbonate, that react and precipitate out. The resulting calcium carbonate precipitate is benign to health, and can be filtered or settled out, yielding low ionic strength product water. The ion exchange and precipitation employs self-sharpening interfaces induced by movement of multiple ions in an electric field to prevent contamination of the product water. Simultaneously, the electrolysis associated with the electromigration produces hydrogen gas, chlorine gas, and sodium hydroxide. We conducted an experimental study of this method's basic efficacy to desalinate salt water from 100 to 600 mol m−3 sodium chloride. We also present physicochemical models of the process, and analyze replacement reagents consumption, permeate recovery ratio, and energy consumption. We hypothesize that the precipitate can be recycled back to replacement reagents using the well-known, commercially implemented Solvay process. We show that the method's permeate recovery ratio is 58% to 46%, which is on par with that of reverse osmosis. We show that the method's energy consumption requirement over and above that necessary to generate electrolysis is 3 to 10 W h l−1, which is on par with the energy consumed by state-of-the-art desalination methods. Furthermore, the method operates at ambient temperature and pressure, and uses no specialized membranes. The process may be feasible as a part of a desalination-co-generation facility: generating fresh water, hydrogen and chlorine gas, and sodium hydroxide.

 Please wait while we load your content...
Please wait while we load your content...