Hydrogen peroxide electrochemistry on platinum: towards understanding the oxygen reduction reaction mechanism†
Abstract
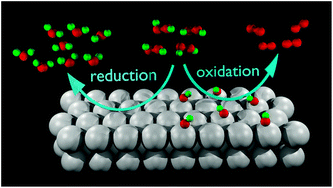
Understanding the hydrogen peroxide electrochemistry on platinum can provide information about the oxygen reduction reaction mechanism, whether H2O2 participates as an intermediate or not. The H2O2

 Please wait while we load your content...
Please wait while we load your content...