Modern battery electrolytes: Ion–ion interactions in Li+/Na+ conductors from DFT calculations†
Abstract
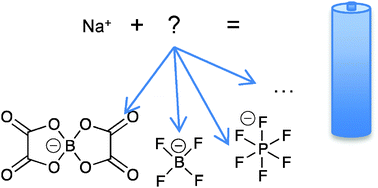
Sodium-ion batteries, the sodium counterpart of the ubiquitous lithium-ion batteries, are currently being developed as a complementary technology to assure resource availability. As battery electrolytes tend to be one of the more limiting parts of any battery for both performance and life-length, chemical and physical data on sodium-ion battery electrolytes are important for rational development. Here the

 Please wait while we load your content...
Please wait while we load your content...