The role of hydrogen bonding in excited state intramolecular charge transfer
Abstract
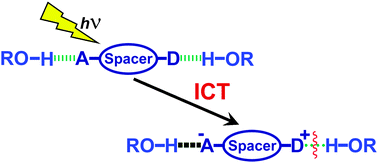
Intramolecular charge transfer (ICT) that occurs upon photoexcitation of molecules is a vital process in nature and it has ample applications in chemistry and biology. The ICT process of the excited molecules is affected by several environmental factors including polarity, viscosity and hydrogen bonding. The effect of polarity and viscosity on the ICT processes is well understood. But, despite the fact that hydrogen bonding significantly influences the ICT process, the specific role of hydrogen bonding in the formation and stabilization of the ICT state is not unambiguously established. Some literature reports predicted that the hydrogen bonding of the solvent with a donor promotes the formation of a twisted intramolecular charge transfer (TICT) state. Some other reports stated that it inhibits the formation of the TICT state. Alternatively, it was proposed that the hydrogen bonding of the solvent with an acceptor favors the TICT state. It is also observed that a dynamic equilibrium is established between the free and the hydrogen bonded ICT states. This perspective focuses on the specific role played by hydrogen bonding of the solvent with the donor and the acceptor, and by proton transfer in the ICT process. The utility of such influence in molecular recognition and anion sensing is discussed with a few recent literature examples in the end.
- This article is part of the themed collection: Hydrogen bonding in electronically excited states

 Please wait while we load your content...
Please wait while we load your content...