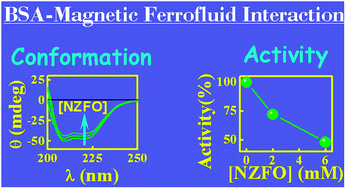
The understanding of the interaction of nanomaterials with relevant biological targets e.g., proteins is of paramount importance in biological and pharmaceutical fields of research. In a biological fluid, proteins can associate with nanomaterials which can subsequently exert a significant impact on the conformation and functionality of the protein. Here we report the binding interaction of a model plasma protein Bovine Serum Albumin (BSA) with a magnetic nanoparticle of mixed spinel origin (Ni0.5Zn0.5Fe2O4, abbreviated as NZFO from now and onwards). The thermodynamic parameters (ΔH, ΔS and ΔG) for the protein–nanoparticle binding interaction have been evaluated from the van't Hoff equation to unveil that the binding interaction is enthalpically as well as entropically driven (ΔH < 0 and ΔS > 0), with an overall favorable Gibbs free energy change (ΔG < 0). Also the thermodynamic parameters delineate the predominant role of electrostatic interaction in the BSA–NZFO binding process. The results of temperature dependent fluorescence quenching and time-resolved fluorescence decay measurements indicate a static quenching mechanism in the present case. Steady-state absorption, synchronous fluorescence, three-dimensional (3D) fluorescence and circular dichroism (CD) spectroscopic techniques have been employed to unveil the conformational changes in BSA induced by the binding of NZFO. Disruption of the native conformation of the protein upon binding with NZFO is reflected through a reduced functionality (in terms of esterase activity) of the protein–NZFO conjugate system in comparison to the native protein. Based on the experimental findings the probable binding location of NZFO is argued to be the hydrophilic domain IB. This seems physically realizable since domain I of BSA is characterized by a net negative charge and hence can serve as a favorable binding site for NZFO carrying a positive surface charge. The key role of electrostatic forces in the BSA–NZFO interaction process is further substantiated from chemical denaturation study and measurement of the effect of ionic strength on the interaction process.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...