When standard reversible potentials for bulk solution reactions, U0, are known, the reversible potentials when the reactant and product are adsorbed on an electrocatalyst surface, Urevsurf, are given in terms of these potentials and the adsorption Gibbs energy bond strengths:Urevsurf = U0 + ΔadsG (Ox)/F − ΔadsG (R)/F
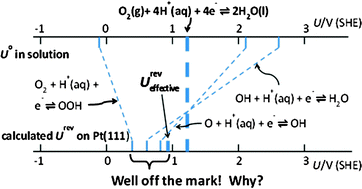
When the ΔadsG (Ox) and ΔadsG (Red) values are known at potential Urevsurf, this equation is exact. When the overpotential for a multi-electron transfer reaction is minimal, each electron transfer takes place at the standard reversible potential for the overall reaction. In the case of O2 reduction to water via the intermediate step OOH(aq) → O(aq) + OH(aq), or via O2(g) → 2O(aq), the respective endergonic O–O dissociation Gibbs energies are shown to be 2.52 eV and 4.76 eV. When the oxygen product and water reactant adsorb weakly, as on platinum, the adsorption Gibbs energies, ΔadsG, for O, OH, and OOH intermediates can be uniquely predicted using these data. All of the above depend exclusively on experimentally determined data. Reversible potentials have been calculated for oxygen reduction steps on the platinum electrocatalyst surface using Interface 1.0, a comprehensive computational code for the potential dependence of the electrochemical interface. Using these results as benchmarks, eqn (i) is found to be accurate to around 0.1 V when the ΔadsG are values calculated for the potentials of zero charge, instead of 1.229 V, which is a significant simplification. The variation in ΔadsG values between the calculated potentials of zero charge and 1.229 V are found to be 0.2 eV V−1 or less. Prior work, using internal adsorption energies calculated at the potential of zero charge in place of Gibbs energies in eqn (i) was found to be accurate to within about 0.2 V. On platinum ΔadsG of the reaction OOH(ads) → O(ads) + OH(ads) is calculated at the potential of zero charge for the reactant and product to be about 1.2 eV exergonic under Langmuir conditions, and this Gibbs energy loss reduces the 1.229 V four-electron reversible potential on the platinum surface to an effective reversible potential of about 0.93 V for this mechanism on platinum. The effective reversible potential is a consequence of efficiency loss, not kinetics. Based on these values, the onset potential for four-electron oxygen reduction will be less than or equal to the effective reversible potential and on pure Pt(111) it appears to be equal to it.

 Please wait while we load your content...
Please wait while we load your content...