Physicochemical properties determined by ΔpKa for protic ionic liquids based on an organic super-strong base with various Brønsted acids†
Abstract
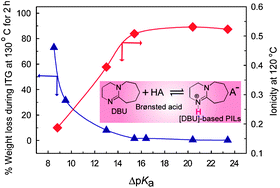
Neutralization of an organic super-strong base, 1,8-diazabicyclo-[5,4,0]-undec-7-ene (DBU), with different Brønsted acids affords a novel series of protic ionic liquids (PILs) with wide variations in the ΔpKa of the constituent amine and acids. The physicochemical properties of these PILs, such as thermal properties, density, conductivity, viscosity, self-diffusion coefficient, vibrational stretching frequency, and 1H-chemical shifts of the N–H bond, have been studied in detail. The generated PILs have melting temperatures below 100 °C, and six are liquids at ambient temperatures. Thermogravimetric analyses (TGA) conducted under isothermal and programmed heating conditions have shown that PILs with ΔpKa ≥ 15 exhibit good thermal stability similar to aprotic ionic liquids. For instance, PILs with ΔpKa > 20 show remarkably high short-term thermal stability up to ca. 450 °C under a nitrogen atmosphere. The viscosity, ionic conductivity, and molar conductivity of the PILs fit well with the Vogel–Fulcher–Tamman equation for their dependencies on temperature. The relative cationic and anionic self-diffusion coefficients of the PILs estimated by the pulsed-field gradient spin-echo (PGSE) NMR method appear to be dependent on the structure and strength of the Brønsted acids. Evaluation of the ionicity based on both the Walden plot and PGSE-NMR revealed that it increases until ΔpKa becomes 15 for the PILs.
- This article is part of the themed collection: Interfaces of Ionic Liquids

 Please wait while we load your content...
Please wait while we load your content...