Tin oxide-surface modified anatase titanium(iv) dioxide with enhanced UV-light photocatalytic activity
Abstract
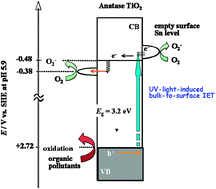
[Sn(acac)2]Cl2 is chemisorbed on the surfaces of anatase TiO2via ion-exchange between the complex ions and H+ released from the surface Ti–OH groups without liberation of the acetylacetonate

 Please wait while we load your content...
Please wait while we load your content...