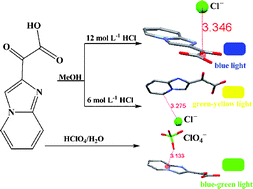
We herein report a new electron-deficient π system, namely carboxycarbonyl substituted imidazo[1,2-a]pyridinium, which exhibits various noncovalent interactions (hydrogen bonding, anion–π and η1-type anion–π) with chloride or perchlorate anion. Crystallographic results demonstrate that the interaction types of chloride anion and π receptor systems can be tuned: from anion–π interactions (with guest methanol molecule in crystal) to η1-type anion–π interactions (without guest methanol molecule in crystal). These carboxycarbonyl substituted imidazo[1,2-a]pyridinium salts also exhibit different phosphorescent colors in the solid state induced by crystal stacking structures or anion–π interactions that can influence the charge transfer to electron-deficient carboxycarbonyl substituted imidazo[1,2-a]pyridinium. Especially, the solid phosphorescent color changes induced by anion–π interactions are very interesting. The high solubility in water makes these purely organic phosphorescent materials promising candidates for potential applications.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...