Tuning the leaving group in 2-deoxy-2-fluoroglucoside results in improved activity-based retaining β-glucosidase probes†
Abstract
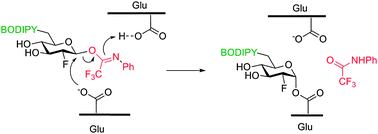
The potency of 2-deoxy-2-fluoroglycosides in activity-based profiling of human acid β-glucosidase is drastically improved by introducing an N-phenyl trifluoroacetimidate leaving group at the anomeric center. Protonation by the general acid–base

 Please wait while we load your content...
Please wait while we load your content...