Nitric oxide coupling mediated by iron porphyrins: the N–N bond formation step is facilitated by electrons and a proton†
Abstract
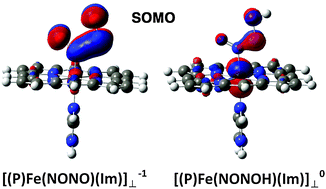
The coupling of two NO molecules catalyzed by iron porphyrins is of biological importance. We use density functional theory calculations to examine the factors that control the fundamental N–N bond formation step mediated by a single iron porphyrin. The presence of an axial Im

 Please wait while we load your content...
Please wait while we load your content...