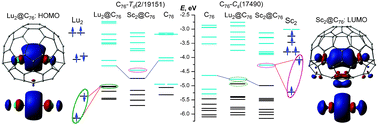
Endohedral metallofullerenes (EMFs) are able to encapsulate up to four metal atoms. In EMFs, metal atoms are positively charged because of the electron transfer from the endohedral metal atoms to the carbon cage. It results in the strong Coulomb repulsion between the positively charged ions trapped in the confined inner space of the fullerene. At the same time, in many EMFs, such as Lu2@C76, Y2@C79N, M2@C82 (M = Sc, Y, Lu, etc.), Y3@C80, or Sc4O2@C80, metals do not adopt their highest oxidation states, thus yielding a possibility of the covalent metal–metal bonding. In some other EMFs (e.g., La2@C80), metal–metal bonding evolves as the result of the electrochemical or chemical reduction, which leads to the population of the metal-based LUMO with pronounced metal–metal bonding character. This article highlights different aspects of the metal–metal bonding in EMFs. It is concluded that the valence state of the metal atoms in dimetallofullerenes is not dependent on their third ionization potential, but is determined by their ns2(n − 1)d1 → ns1(n − 1)d2 excitation energies. Peculiarities of the metal–metal bonding in EMFs are described in terms of molecular orbital analysis as well as topological approaches such as Quantum Theory of Atoms in Molecules and Electron Localization Function. Interplay of Coulomb repulsion and covalent bonding is analyzed in the framework of the Interacting Quantum Atom approach.

 Please wait while we load your content...
Please wait while we load your content...