Structural basis for oxygen sensing and signal transduction of the heme-based sensor protein Aer2 from Pseudomonas aeruginosa†‡
Abstract
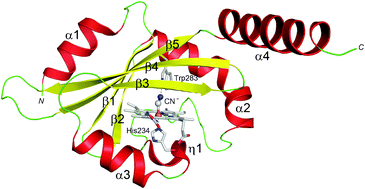
The crystal structure of a truncated Aer2, a signal transducer
- This article is part of the themed collection: Porphyrins & Phthalocyanines

 Please wait while we load your content...
Please wait while we load your content...