Organic base-catalyzed stereodivergent synthesis of (R)- and (S)-3-amino-4,4,4-trifluorobutanoic acids†‡
Abstract
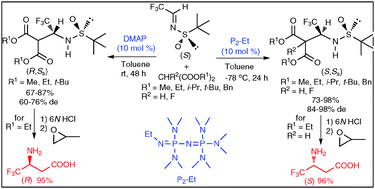
Organic base-catalyzed reaction of (S)-N-tert-butanesulfinyl (3,3,3)-trifluoroacetaldimine with dialkyl malonates was found to be effective for synthesis of both (S,SS) and (R,SS) β-aminomalonates in high yield with good to excellent diastereoselectivity (76–98% de). The products of this Mannich reaction provide direct access to
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...