An ab initio mechanism for efficient population of triplet states in cytotoxic sulfur substituted DNA bases: the case of 6-thioguanine†
Abstract
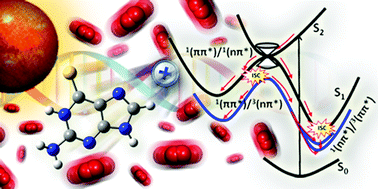
The deactivation mechanism of the cytotoxic 6-thioguanine, the 6-sulfur-substituted analogue of the canonical DNA base, is unveiled by ab initio calculations. Oxygen-by-sulfur substitution leads to efficient population of triplet states—the first step for generating singlet oxygen—which is responsible for its cytotoxicity.

 Please wait while we load your content...
Please wait while we load your content...