The isolation of a metastable conglomerate using a combined computational and controlled crystallization approach
Abstract
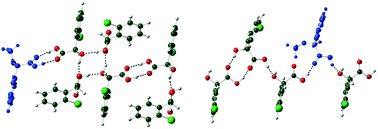
While the use of additives to control the crystallization of polymorphs is well known, similar methodology to promote the crystallization of a metastable conglomerate over a stable racemic compound in enantiomeric systems has not been reported. Here we demonstrate this phenomenon in the case of

 Please wait while we load your content...
Please wait while we load your content...