Understanding the regioselectivity in Scholl reactions for the synthesis of oligoarenes†
Abstract
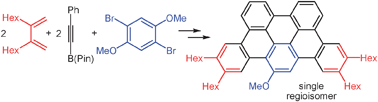
A short reaction sequence leads to oligoarene derivatives utilising a regioselective Scholl reaction for the unprecedented cyclisation to the mono-functionalised oligoarene under methanol elimination. Quantum-chemical investigations reveal the reason for the remarkably high regioselectivity.

 Please wait while we load your content...
Please wait while we load your content...