Sulfo-click reaction via in situ generated thioacids and its application in kinetic target-guided synthesis†‡
Abstract
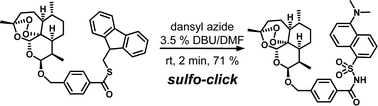
Herein, we describe a practical, one-pot variant of the sulfo-click reaction, in which 9-fluorenylmethyl-protected
- This article is part of the themed collection: Emerging Investigators 2012

 Please wait while we load your content...
Please wait while we load your content...