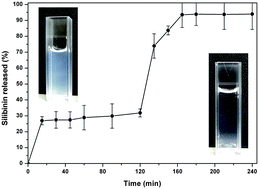
Orally administered bioactive compounds have to survive acidic environs (pH 1.2) of the stomach and reach the small intestine (pH 6.8–7.4), which is the main site of absorption in the gastrointestinal tract. Hence, it is desired to have a delivery system which can protect the active molecules from degradation in acidic pH but release them in the small intestine. This objective was achieved in the present work by formulating stable shellac colloidal particles that showed pH dependent release. Colloidal particles were prepared using a simple anti-solvent method, wherein shellac was precipitated spontaneously by adding ethanolic shellac solution to aqueous polymer (xanthan gum) solution, which acts as electro-steric stabiliser. The average particle size could be controlled from around 150 nm to 300 nm depending on the initial concentration of shellac in the stock solution. High negative surface charge (−64.8 to −81.7 mV) and spherical shape of the particles were confirmed using zeta-sizer and transmission electron microscopy. To evaluate the suitability of the colloidal particles for delivery of bioactive compounds, silibinin (a flavonolignan) was loaded up to 10% w/w into the shellac particles and characterised for pH stability and in vitro release. X-Ray diffraction studies indicated amorphous nature of the entrapped silibinin in the colloidal particles. The release of silibinin was followed under gastric (pH 1.2) and intestinal conditions (pH 7.4) and the amount was detected in terms of antioxidant activity (Ferric Reducing Antioxidant Power). Due to the pH dependent solubility of shellac, silibinin entrapped in the colloidal particles was found to be stable to acidic pH and showed more than 90% release in the intestinal pH.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...