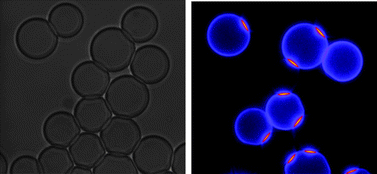
Bi-dimensional (2D) specific interactions are crucial in numerous biological processes. Attachment of molecules to a surface provides several distinct properties absent in a three-dimensional (3D) configuration, such as type of surface molecular anchorage, nature of the surface and sensitivity to local mechanical stress. While rules governing formation and stability of specific molecular interactions in 3D have been extensively described, much less is known about 2D. Here, we devised a model experiment seeking to clarify the main features of these interactions when molecules are attached to liquid interfaces. We prepared two complementary micrometric liquid droplet populations exhibiting either streptavidin as the receptor or biotin as the ligand. We brought the grafted interfaces into contact under different experimental configurations and analyzed the molecular interfacial contact formed. We measured contact formation characteristic time, molecular density, molecular dynamics and geometry. We observed formation of an irreversible liquid film concentrated in ligands and receptors and driven by specific molecular interactions. Molecular contact, although sterically saturated, was not frozen and the bonds themselves were capable of lateral diffusion in the confined space of interdroplet film. While bond diffusion was limited to the interfacial contact zone, unbound molecules were free to be exchanged between droplet contact and contour. We concluded that formation of specific receptor–ligand bonds between liquid interfaces resulted from the subtle interplay of universal surface forces and specific molecular interactions. This shed light on an interesting system of functional colloidal interactions in which molecular composition and physical properties of interfacial contact could be independently tuned.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...