Synthesis and characterization of a cyclic vinylpalladium(ii) complex: vinylpalladium species as the possible intermediate in the catalytic direct olefination reaction of enamide†
Abstract
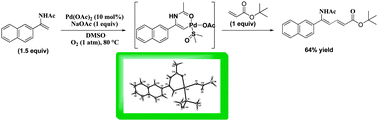
A six-membered cyclic vinylpalladium complex was synthesized via

 Please wait while we load your content...
Please wait while we load your content...