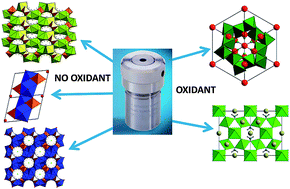
We report the results of an exploratory synthetic study of iridium-containing materials using hydrothermal methods from simple metal salts. Three alkali-earth iridium hydroxides are isolated as phase-pure samples and their structures examined by single-crystal or powder diffraction methods: each contains Ir(IV)-centred octahedra, isolated from each other and sharing bridging hydroxides or fluoride with alkali-earth (Ca, Sr or Ba) centres. One of these hydroxides, Ca2IrF(OH)6.OH, possesses a unique open structure, consisting of a positively-charged framework that has one-dimensional channels in which infinite chains of hydrogen-bonded hydroxide anions are encapsulated. The addition of hydrogen peroxide or sodium peroxide to otherwise identical hydrothermal reactions yields dense oxide materials in which iridium is found in an oxidation state between +4 and +5: the novel oxide Na0.8Sr2.2Ir3O10.1 has a KSbO3-type structure with an iridium oxidation state of +5, while the new pyrochlore (Na0.27Ca0.59)2Ir2O6·0.66H2O contains iridium with an average oxidation state close to +4.5. Our results illustrate the utility for hydrothermal synthesis in the discovery of novel complex structures that may be inaccessible using conventional high-temperature synthesis, with control of the metal oxidation state possible with judicious choice of reagents.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...