Simple and efficient copper metal-mediated synthesis of alkoxyamine initiators†
Abstract
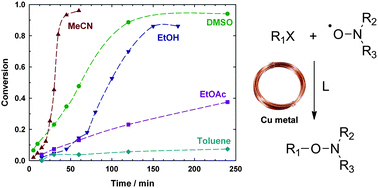
A simple and efficient method is presented for the preparation of a wide range of alkoxyamines from nitroxide radicals and activated alkyl bromides at room temperature. The reaction requires a stoichiometric amount of copper metal (0.5 mol mol−1 alkyl bromide) and proceeds most rapidly in

 Please wait while we load your content...
Please wait while we load your content...