Enantiospecific 6π-photocyclization of atropisomeric α-substituted acrylanilides in the solid-state: role of crystalline confinement on enantiospecificity†‡
Abstract
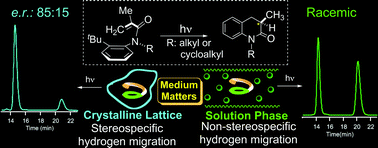
Direct irradiation of optically-pure axially-chiral α-substituted acrylanilides in the crystalline state leads to 3,4-dihydro-2-quinolin-2-one photoproduct with an enantiomeric ratio of 85 : 15 while a racemic mixture of photoproduct is observed in solution.
- This article is part of the themed collection: In honour of the contribution of Japanese scientists to photochemistry

 Please wait while we load your content...
Please wait while we load your content...