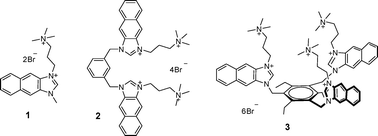
Naphthoimidazolium groups can form unique ionic hydrogen bonds with anions as imidazolium moieties, and in addition, they are fluorescent, so no further elaborative synthesis is needed to introduce a fluorescent group. In this paper, three naphthoimidazolium derivatives were synthesized and studied for the recognition of nucleotides. Compound 1 composed of a single naphthoimidazolium group and quaternary ammonium group did not show any significant fluorescent changes with various anions and nucleotides, such as ATP, GTP, CTP, TTP, UTP, ADP and AMP. A tripodal compound 3 bearing three naphthoimidazolium groups and three quaternary ammonium groups, respectively, showed large fluorescence enhancements with UTP, CTP and TTP and moderate fluorescence enhancements with ATP and pyrophosphate and a fluorescence quenching effect with GTP. On the other hand, compound 2 bearing two naphthoimidazolium groups and two quaternary ammonium groups displayed a selective fluorescence enhancement with ATP and a selective fluorescence quenching effect with GTP in 100% aqueous solution.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...