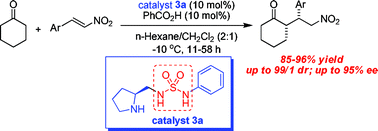
Pyrrolidinyl-sulfamide derivatives as a new class of bifunctional organocatalysts for direct asymmetric Michael addition of cyclohexanone to nitroalkenes†
Abstract
A series of chiral pyrrolidinyl-sulfamide derivatives have been identified as efficient bifunctional organocatalysts for the direct Michael addition of
 Please wait while we load your content...
Please wait while we load your content...