trans,trans-2,4-Hexadiene incorporation on enzymes for site-specific immobilization and fluorescent labeling†
Abstract
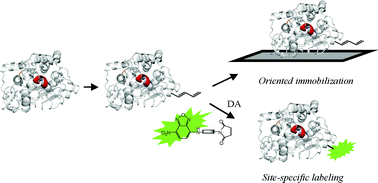
Lipase B from Candida antarctica (CAL-B) has been site-directedly modified by the introduction of a trans,trans-hexadiene moiety onto lipase molecules, identified by MALDI-TOF. This modification on CAL-B permitted its immobilization on Q-Sepharose supports in excellent yields (>95%) when native lipase was not immobilized at pH 7 and 25 °C. After the entire modification procedure, the catalytic activity of the
- This article is part of the themed collection: Chemical Biology

 Please wait while we load your content...
Please wait while we load your content...