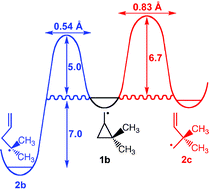
CVT + SCT calculations on the rate of tunnelling at 20 K in the ring opening of cyclopropylcarbinyl radical, substituted with geminal methyl groups at a ring carbon (1b), have been performed. The calculations predict that, contrary to expectations based on the effect of mass on the rate of tunnelling, the geminal methyl substituents in 1b should make the rate of ring opening to 1,1-dimethyl-3-butenyl radical (2b) 104 times faster than the rate of ring opening of unsubstituted cyclopropylcarbinyl radical (1a) to 3-butenyl radical (2a) and almost 106 times faster than the rate of ring opening of 1b to 2,2-dimethyl-3-butenyl radical (2c). The reasons for these unexpected findings are discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...