Novel pyrrolidine-thiohydantoins/thioxotetrahydropyrimidinones as highly effective catalysts for the asymmetric Michael addition†
Abstract
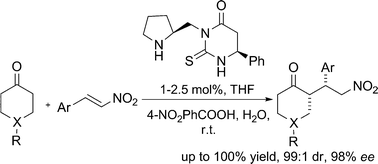
The synthesis of novel organocatalysts consisting of a pyrrolidine moiety and a thiohydantoin or a thioxotetrahydropyrimidinone ring is described. The compound combining the

 Please wait while we load your content...
Please wait while we load your content...