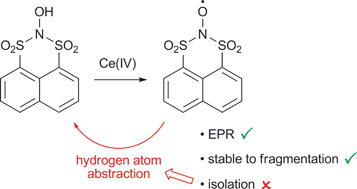
Acyclic bissulfonylnitroxides have never been isolated, and degrade through fragmentation. In an approach to stabilising a bissulfonylnitroxide radical, the cyclic, peri-substituted N,N-bissulfonylhydroxylamine, 2-hydroxynaphtho[1,8-de][1,3,2]dithiazine 1,1,3,3-tetraoxide (1), has been prepared by formal nitrogen insertion into the sulfur–sulfur bond of a sulfinylsulfone, naphtho[1,8-cd][1,2]dithiole 1,1,2-trioxide. The heterocyclic ring of 1 is shown to adopt a sofa conformation by X-ray crystallography, with a pseudo-axial hydroxyl group. N,N-Bissulfonylhydroxylamine 1 displays high thermal, photochemical and hydrolytic stability compared to acyclic systems. EPR analysis reveals formation of the corresponding bissulfonylnitroxide 2 upon oxidation of 1 with the Ce(IV) salts CAN and CTAN. Although 2 does not undergo fragmentation, it cannot be isolated, since hydrogen atom abstraction to reform 1 occurs in situ. The stability and reactivity of 1 and 2 are compared with the known cyclic benzo-fused N,N-bissulfonylhydroxylamine, N-hydroxy-O-benzenedisulfonimide (6), for which the X-ray data, and EPR of the corresponding nitroxide 10, are also reported for the first time.

 Please wait while we load your content...
Please wait while we load your content...