Efficient total synthesis of (−)-stemoamide†
Abstract
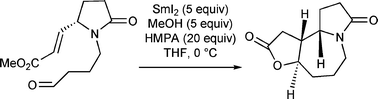
An efficient diastereoselective synthesis of (−)-stemoamide has been accomplished from a pyroglutamic acid derivative in eight steps and with 24% overall yield. The synthesis features an intramolecular samarium diiodide-promoted 7-exo-trigcyclization of a ketyl radical generated from the corresponding

 Please wait while we load your content...
Please wait while we load your content...