Abstract
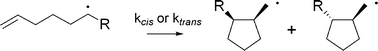
Rate constant data and Arrhenius parameters have been determined for a series of substituted hexenyl radicals of differing electronic and steric demand. Electron-withdrawing groups (CF3, CO2Et) directly attached to the radical centre slighly accelerate 5-exo ring-closure (kcis + ktrans ∼ 2.1 × 105 s−1 at 25°) relative to donating groups (OMe; 1.6 × 105 s−1 at 25°). Sterically demanding groups (tert-Bu), as expected, slow the cyclization process (1 × 105 s−1). These observations are consistent with subtle changes in
- This article is part of the themed collection: Free Radical Chemistry special themed issue in memory of Athel Beckwith

 Please wait while we load your content...
Please wait while we load your content...