The Grob/Eschenmoser fragmentation of cycloalkanones bearing β-electron withdrawing groups: a general strategy to acyclic synthetic intermediates†
Abstract
Introduction of a β-electron withdrawing group to cycloalkanones allows facile C–C bond fragmentation. The reaction has been demonstrated with a large range of ring sizes, bearing various leaving and electron withdrawing groups, and using a variety of
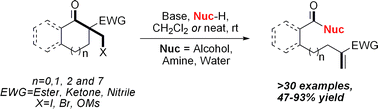

 Please wait while we load your content...
Please wait while we load your content...