Guanidiniocarbonyl-pyrrole-aryl conjugates as nucleic acid sensors: switch of binding mode and spectroscopic responses by introducing additional binding sites into the linker†
Abstract
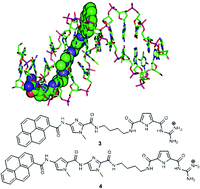
Two novel guanidiniocarbonyl pyrrole–
- This article is part of the themed collection: Chemical Biology

 Please wait while we load your content...
Please wait while we load your content...