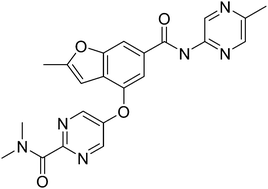
Designing glucokinase activators with reduced hypoglycemia risk: discovery of N,N-dimethyl-5-(2-methyl-6-((5-methylpyrazin-2-yl)-carbamoyl)benzofuran-4-yloxy)pyrimidine-2-carboxamide as a clinical candidate for the treatment of type 2 diabetes mellitus
J. A. Pfefferkorn, A. Guzman-Perez, P. J. Oates, J. Litchfield, G. Aspnes, A. Basak, J. Benbow, M. A. Berliner, J. Bian, C. Choi, K. Freeman-Cook, J. W. Corbett, M. Didiuk, J. R. Dunetz, K. J. Filipski, W. M. Hungerford, C. S. Jones, K. Karki, A. Ling, J. Li, L. Patel, C. Perreault, H. Risley, J. Saenz, W. Song, M. Tu, R. Aiello, K. Atkinson, N. Barucci, D. Beebe, P. Bourassa, F. Bourbounais, A. M. Brodeur, R. Burbey, J. Chen, T. D'Aquila, D. R. Derksen, N. Haddish-Berhane, C. Huang, J. Landro, A. Lee Lapworth, M. MacDougall, D. Perregaux, J. Pettersen, A. Robertson, B. Tan, J. L. Treadway, S. Liu, X. Qiu, J. Knafels, M. Ammirati, X. Song, P. DaSilva-Jardine, S. Liras, L. Sweet and T. P. Rolph,
Med. Chem. Commun., 2011, 2, 828
DOI: 10.1039/C1MD00116G
To request permission to reproduce material from this article, please go to the
Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission
provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures
and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article
in a third-party publication (excluding your thesis/dissertation for which permission is not required)
please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.

 Please wait while we load your content...
Please wait while we load your content...