Triazole-substituted N-hydroxyindol-2-carboxylates as inhibitors of isoform 5 of human lactate dehydrogenase (hLDH5)†
Abstract
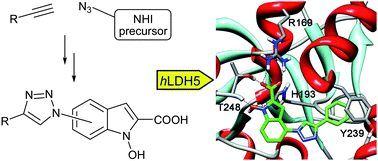
hLDH5 plays a key role in the glycolytic metabolic switch found in invasive tumours and is now considered as a promising therapeutic target in cancer. Here we report a series of new hLDH5-inhibitors based on triazole-containing N-hydroxyindol-2-carboxylates, whose synthesis includes the highly reliable and simple “click chemistry” reaction.

 Please wait while we load your content...
Please wait while we load your content...