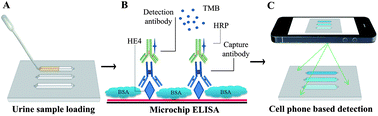
Ovarian cancer is asymptomatic in the early stages and most patients present with advanced levels of disease. The lack of cost-effective methods that can achieve frequent, simple and non-invasive testing hinders early detection and causes high mortality in ovarian cancer patients. Here, we report a simple and inexpensive microchip ELISA-based detection module that employs a portable detection system, i.e., a cell phone/charge-coupled device (CCD) to quantify an ovarian cancer biomarker, HE4, in urine. Integration of a mobile application with a cell phone enabled immediate processing of microchip ELISA results, which eliminated the need for a bulky, expensive spectrophotometer. The HE4 level detected by a cell phone or a lensless CCD system was significantly elevated in urine samples from cancer patients (n = 19) than healthy controls (n = 20) (p < 0.001). Receiver operating characteristic (ROC) analyses showed that the microchip ELISA coupled with a cell phone running an automated analysis mobile application had a sensitivity of 89.5% at a specificity of 90%. Under the same specificity, the microchip ELISA coupled with a CCD had a sensitivity of 84.2%. In conclusion, integration of microchip ELISA with cell phone/CCD-based colorimetric measurement technology can be used to detect HE4 biomarker at the point-of-care (POC), paving the way to create bedside technologies for diagnostics and treatment monitoring.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...