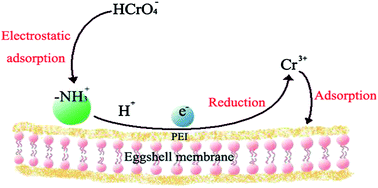
The polyethyleneimine (PEI) functionalized biosorbent which uses an eggshell membrane (ESM) as a model was synthesized based on the cross linking reaction between aldehydes in glutaraldehyde and functional groups such as amines and amides in ESM. The as-prepared biosorbent (PEI–ESM) strongly interacted with chromium(VI). After modification, the dynamic uptake capacity of the PEI–ESM increased by 105% compared with the control, and the maximum equilibrium adsorption capacity for Cr(VI) can reach about 160 mg g−1 with an initial pH of 3.0. The Langmuir adsorption isotherm was applicable to fit the removal process. Kinetics of the Cr(VI) removal were found to follow a pseudo-second-order rate equation. The results obtained by Raman spectroscopy and X-ray photoelectron spectroscopic analysis (XPS), performed on the as-prepared biosorbent before and after Cr(VI) adsorption, suggested that some of adsorbed Cr(VI) anions were reduced to Cr(III) in Cr2O3 or Cr(OH)3 during the sorption process, demonstrating that the PEI–ESM could detoxify Cr(VI). The developed biosorbent promises advantages such as low cost, high adsorption capacity, Cr(VI) detoxification, and environmental friendliness.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...