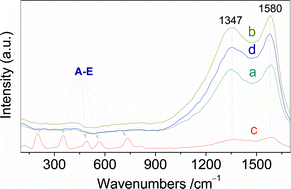
An adduct behaviour of ammonium molybdate tetrahydarte (AMT) with polytetrafluoroethylene (PTFE) resulted in three significant benefits: (a) a much lower degradation temperature (ΔTm, 82 K) of the PTFE in the adduct and a much lower final residual mass (ΔRM, 39%) of the adduct compared to the theoretical prediction, (b) the formation of MoO3 and MoO2 nanoparticles, and (c) the shuttle-shaped MoO2 nanoparticles coated with graphite exhibits superior soft magnetic property. The much earlier degradation suggested that there was a strong mutual effect between AMT and PTFE. The much lower residual mass implied that the intervention of a chemical reaction was responsible for the effect in nitrogen. Actually, Raman spectra revealed that the adducted PTFE was degraded into a graphite structure at this atmosphere, and the presence of the graphite layer led to the reduction of AMT into shuttle-shaped MoO2 nanoparticles at 837 K to a complete extent. Controlled sintering measurements in air indicated that the AMT in the presence of PTFE produced α-MoO3 nanoparticles, instead of MoO2. Further, our results indicated that the MoO3 nanoparticles obtained had a positive response to the heterogeneous catalytic oxidation of thiophene in the presence of hydrogen peroxide. We believe that the present work is not only of relevance for applications in the degradation of polymer materials, but also will attract the attention of many groups studying the preparation and magnetic properties of transition metal oxide nanoparticles and their practical application in heterogeneous catalytic reactions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...