A stable electrochemically active copper interface for room-temperature ionic liquidvia surface modification to a metal–organic charge-transfer complex
Abstract
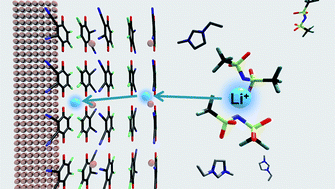
A novel electrochemical interface between metal electrode and room-temperature
* Corresponding authors
a
Institute of Multidisciplinary Research for Advanced Materials (IMRAM), Tohoku University, Katahira 2-1-1, Aoba-ku, Sendai, Japan
E-mail:
i.honma@tagen.tohoku.ac.jp
Fax: +81 22 217 5828
Tel: +81 22 217 5816
A novel electrochemical interface between metal electrode and room-temperature

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
Y. Hanyu and I. Honma, J. Mater. Chem., 2011, 21, 9154 DOI: 10.1039/C1JM00026H
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
