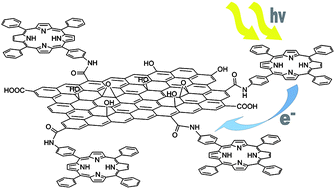
The successful covalent functionalization of graphene oxide (GO) with 5-(4-aminophenyl)-10,15,20-triphenyl-21,23H-porphyrin (H2P) has been reported. The resulting GO–H2P hybrid material forms stable dispersions in DMF and has been thoroughly characterized by spectroscopic (UV-vis, ATR-IR, Raman) and thermal (TGA) tools. Microscopy techniques (AFM and TEM) have been employed to probe the morphological characteristics as well as to investigate the exfoliation of graphene sheets. Steady-state and time-resolved fluorescence emission studies have shown efficient fluorescence quenching, suggesting that electron transfer occurs from the singlet excited state of the H2P moiety to the GO sheet. Photoexcitation resulted in the one-electron oxidation of the H2P with the simultaneous one-electron reduction of GO, yielding (GO)˙−–(H2P)˙+, as revealed by transient absorption measurements. The electrochemical redox potentials of the graphene–H2P material were studied by cyclic voltammetry (CV) and differential pulsed voltammetry (DPV) and the energy gap for the charge-separated state of (GO)˙−–(H2P)˙+ has been calculated as 0.87 eV, while, the negative free-energy change for the photoinduced charge-separation of GO–H2P has been evaluated to be −1.00 eV, which confirms the thermodynamically favorable formation of (GO)˙−–(H2P)˙+. Eventually, the GO–H2P hybrid material was deposited by electrophoretic deposition onto SnO2 electrodes, and the OTE/SnO2/GO–H2P electrode exhibited an incident photon-to-photocurrent-efficiency IPCE of 1.3% in a standard photoelectrochemical cell.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...