The solvent-free and catalyst-free conversion of an aziridine to an oxazolidinone using only carbon dioxide†
Abstract
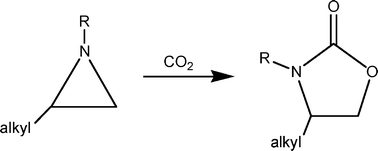
It has been found for the first time at room temperature that the reaction of an unactivated 2-alkyl or 2-aryl aziridine with carbon dioxide to generate the corresponding

 Please wait while we load your content...
Please wait while we load your content...