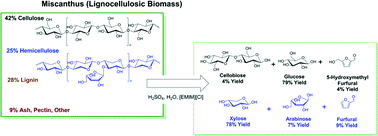
Experiments were conducted to study the effects of reaction conditions on the hydrolysis of miscanthus dissolved in 1-ethyl-3-methylimidazolium chloride ([Emim][Cl]) catalyzed by H2SO4. It was determined that while there is a small co-inhibition effect associated with the simultaneous hydrolysis of the cellulosic and hemicellulosic portions of miscanthus, the largest rate decreases were observed for the hydrolysis of the hemicellulosic portion. This rate decrease was attributed to the chemical linkage between hemicellulose and lignin in miscanthus, which could be broken with chemical pretreatment. While chemical pretreatment increased the rate of the hydrolysis of the hemicellulosic component, delignification showed no further benefit. The rate of hydrolysis was determined to be first order in concentrations of β-1,4 glycosidic linkage and acid, and zero order in water concentration. The activation energy for the hydrolysis of the glycosidic linkages in the cellulosic and hemicellulosic components were determined to be 95 kJ mol−1 and 114 kJ mol−1 respectively. Progressive addition of water during the first hour of the reaction increased conversion and selectivity to saccharine products, while limiting dehydration of the sugars formed. The conversion of the cellulosic portion of miscanthus could be increased after the first hour of the reaction by increasing the reactor temperature. While miscanthus is only partially soluble in [Emim][Cl], it was found that the initial miscanthus loading could be increased to 9 wt% before significant yield decreases attributed to solubility limitations of the cellulosic component were observed. By proper adjustment of reaction conditions, it was possible to achieve yields of sugars approaching 84% from the cellulosic and hemicellulosic components of miscanthus, with minimal dehydration of the sugars to furans.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...