The governing self-discharge processes in activated carbon fabric-based supercapacitors with different organic electrolytes
Abstract
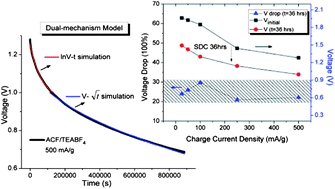
Electrochemical power devices with a long lifespan, long-term energy retention and great cycle stability are extremely important for periodic energy store/supply, especially for solar energy storage for space equipment and for power electronics in integrated circuits. In this report, we have systematically investigated the effects of the charging current density and temperature over the self-discharge (SDC) process of activated carbon fabric-based (ACF) supercapacitors with 1 M LiPF6/EC–DEC (v/v = 1) and 1 M TEABF4/PC as electrolytes, respectively. The experimental results have shown that a different control mechanism governs the SDC process in each electrolyte system. Significant energy retention (in excess of 70%) was obtained in the ACF–TEABF4 system after 36 h. SDC at room temperature. A dual-mechanism control model is proposed for the first time which describes perfectly the SDC process of the supercapacitor using 1 M TEABF4/PC as the electrolyte over different charge current densities and at different SDC temperatures.

 Please wait while we load your content...
Please wait while we load your content...