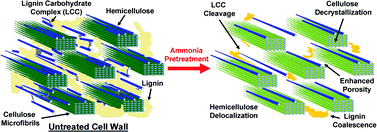
Deconstruction of lignocellulosic plant cell walls to fermentable sugars by thermochemical and/or biological means is impeded by several poorly understood ultrastructural and chemical barriers. A promising thermochemical pretreatment called ammonia fiber expansion (AFEX) overcomes the native recalcitrance of cell walls through subtle morphological and physicochemical changes that enhance cellulase accessibility without extracting lignin and hemicelluloses into separate liquid streams. Multi-scale visualization and characterization of Zea mays (i.e., corn stover) cell walls were carried out by laser scanning confocal fluorescence microscopy (LSCM), Raman spectroscopy, atomic force microscopy (AFM), electron microscopy (SEM, TEM), nuclear magnetic resonance (NMR), and electron spectroscopy for chemical analysis (ESCA) to elucidate the mechanism of AFEX pretreatment. AFEX first dissolves, then extracts and, as the ammonia evaporates, redeposits cell wall decomposition products (e.g., amides, arabinoxylan oligomers, lignin-based phenolics) on outer cell wall surfaces. As a result, nanoporous tunnel-like networks, as visualized by 3D-electron tomography, are formed within the cell walls. We propose that this highly porous structure greatly enhances enzyme accessibility to embedded cellulosic microfibrils. The shape, size (10 to 1000 nm), and spatial distribution of the pores depended on their location within the cell wall and the pretreatment conditions used. Exposed pore surface area per unit AFEX pretreated cell wall volume, estimated via TEM-tomogram image analysis, ranged between 0.005 and 0.05 nm2 per nm3. AFEX results in ultrastructural and physicochemical modifications within the cell wall that enhance enzymatic hydrolysis yield by 4–5 fold over that of untreated cell walls.

 Please wait while we load your content...
Please wait while we load your content...