Rational design of aminoanthraquinones for colorimetric detection of heavy metal ions in aqueous solution†
Abstract
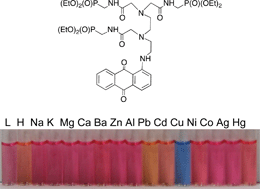
A family of water-soluble colorimetric chemosensors incorporating an
- This article is part of the themed collection: Dalton Transactions 40th Anniversary

 Please wait while we load your content...
Please wait while we load your content...