Chiral palladacycles based on resorcinol monophosphite ligands: the role of the meta-hydroxyl in ligand C–H activation and catalysis†
Abstract
The reactions of a range of chiral
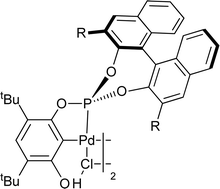
- This article is part of the themed collection: Pincers and other hemilabile ligands

 Please wait while we load your content...
Please wait while we load your content...